Just as chemistry supervenes on physics and biology supervenes on chemistry, BioPrinting supervenes on 3d Printing. For this reason it can trace its origins to the invention of stereo-lithography by inventor Charles Hull in 1984, a technology that uses an additive layering process to render 3d digital designs as 3d physical objects. Once the photo-polymer is cured under UV light, you have a tangible version of whatever you were previously looking at on-screen.
Bio-printing’s first breakthrough occurred in 1996 when scientists Gabor Forgacs and his team began experimenting with live cells. They noticed that the cells had a tendency to organize according to their genetic programming even when outside of the animal. Furthermore the extra-cellular matrix that the cells produced, would initially be liquid and become less so as the cells matured. This window of opportunity allowed the cells to be shaped prior to maturing and was analogous to the UV curing method utilized in materials science. That insight inspired the further development of these technologies.
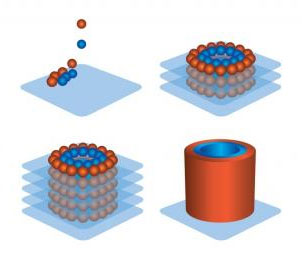
In 2000, a a human bladder was increased in size using a synthetic scaffold in a live patient. Although the cells had not been printed, they had been engineered and “taught” how to behave. When introduced to the synthetic scaffolding they arranged themselves as they would have in nature, immensely decreasing the possibility of rejection. This behavior of cells to behave as if “within the body” and arrange themselves even when grown outside the body in no particular arrangement gained prominence among scientific communities. Subsequently, research teams around the world searched for “killer” applications. Over the next decade entire organs (Hearts, kidneys, and lungs) were grown to functioning states (although not implantable) on collagen scaffolding.

In 2003, a Professor from the University of Texas at Paso outfitted an inkjet printer with a cell deposition unit. This advancement, however rudimentary, proved that an additive printing process could deliver cells onto a scaffold with precision. It was only a year later that Forgacs reemerged with a method of depositing only cells, sans the scaffold, keeping them healthy enough to produce their own extra-cellular matrix and structural proteins.
http://www.wakehealth.edu/Research/WFIRM/Our-Story/Inside-the-Lab/Bioprinting.htm
The technology develops for 5 years and in 2009 Organovo, a company founded by Forgacs, introduces the Novogen MMX BioPrinter, the first market-ready version of their technology. In 2009, they print nervous tissue, followed by blood vessels in 2010, cardiac tissue in 2011, and lung tissue in 2012. Universities, notably Wake Forest, followed suit with their own versions of the technology.
Currently, they are experimenting with printing entire livers and they have reportedly done so with only a 10 day time-frame. It will still be about a decade, however, before they are of sufficient quality that they can be transplanted.